Lien
Laboratory
 Diet, Metabolism and Cancer
Diet, Metabolism and Cancer
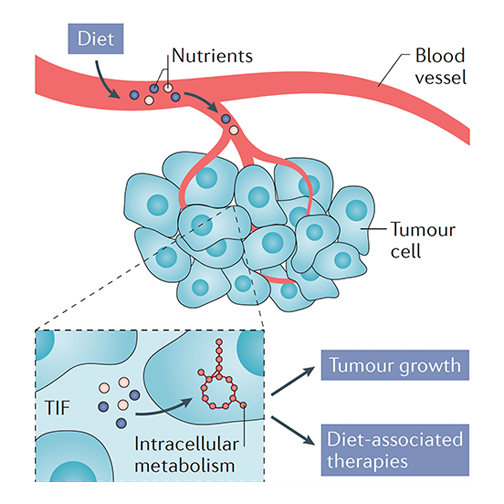
Cancer cells have voracious appetites for nutrients and energy, which they use to fuel their uncontrolled growth and invade healthy tissue. Since diet and nutrition are major determinants of which nutrients are supplied to cancer cells, how diet affects cancer progression is an important question for many cancer patients. While there is no shortage of proposed “anti-cancer diets” in our popular culture, our scientific understanding of this question is limited, highlighting the need for systematic analyses of how different diets alter cancer progression and therapy responses.
By using cancer models together with mass spectrometry, molecular biology and genetic approaches, the Lien Lab assesses how diet-mediated changes to nutrient availability in the tumor microenvironment affect tumor metabolism, progression and responses to therapy. These efforts aim to uncover how variations in a person’s diet may influence tumor growth and metabolism with the goal of translating these findings into actionable strategies for improving cancer treatment and response.
To achieve this goal, the Lien Lab pursues three lines of research:
- Profiling how different diets alter tumor growth and metabolism
- Exploring how metabolic pathways are regulated in response to diet-induced changes to nutrient levels in the tumor microenvironment, with a particular focus on lipid metabolism pathways
- Identifying strategies for using dietary interventions to enhance the efficacies of standard-of-care and emerging cancer therapies
Join Our Team!
The Laboratory of Dr. Evan Lien, with the MNP, is open to speaking with prospective Postdoctoral Fellow candidates about the possibility of joining his research team. If interested, you are welcome to contact Dr. Evan Lien directly.
News & Featured Publications
Learn More
Cancer cells can use backup routes to fuel their growth

Hope in action: How Bee Brave fuels breast cancer breakthroughs at VAI

Starving cancer cells of fat may improve cancer treatment
Lien EC, Westermark AM, Zhang Y, Yuan C, Li Z, Lau AN, Sapp KM, Wolpin BM, Vander Heiden MG. 2021. Low glycaemic diets alter lipid metabolism to influence tumour growth. Nature 599:302–307.
Lien EC, Vander Heiden MG. 2019. A framework for examining how diet impacts tumour metabolism. Nat Rev Cancer. 19(11):651–661.
OUR IMPACT
We’re raising thousands to save millions.
We’re turning hope into action for the millions of people around the world affected by diseases like cancer and Parkinson’s. Find out how you can help us make a difference.
- 122 peer-reviewed papers published in 2024, 63 of which were in high-impact journals
- 15 VAI-SU2C Epigenetics Dream Team clinical trials launched to date
- 10 clinical trials co-funded by VAI & Cure Parkinson's (out of 41 total International Linked Clinical Trials Program trials)
Evan Lien, Ph.D.
Assistant Professor, Department of Metabolism and Nutritional Programming
Areas of Expertise
Metabolism, cancer, tumor microenvironment, nutrition, lipid metabolism
Biography
Dr. Evan Lien studies the molecular and biochemical interactions between diet, metabolism and cancer with the goal of developing new prevention and treatment strategies. He earned his Ph.D. in biological and biomedical sciences from Harvard University under the mentorship of Alex Toker, Ph.D, where he studied how oncogenic PI3K signaling regulates cellular metabolism in breast cancer. Dr. Lien then joined the lab of Matthew G. Vander Heiden, M.D., Ph.D., at MIT’s Koch Institute for Integrative Cancer Research as a postdoctoral fellow. While there, he evaluated how dietary interventions, such as low glycemic diets, impact cancer metabolism, growth and response to therapy. Dr. Lien’s research into the genetic and environmental factors that regulate cancer cell metabolism have earned him numerous accolades, including a Damon Runyon Postdoctoral Fellowship and a National Cancer Institute K99/R00 grant — both highly competitive awards. In 2022, he joined Van Andel Institute’s Department of Metabolism and Nutritional Programming as an assistant professor.
2025
Kaluba FC, Rogers TJ, Jeong YJ, House RJ, Waldhart A, Sokol KH, Daniels SR, Lee CJ, Longo J, Johnson A, Sartori V, Sheldon RD, Jones RG, Lien EC. 2025. An alternative route for β-hydroxybutyrate metabolism supports cytosolic acetyl-CoA synthesis in cancer cells. Nat Metab.
2024
Lien EC, Vu N, Westermark AM, Danai LV, Lau AN, Gültekin Y, Kukurugya MA, Bennett BD, Vander Heiden MG. 2024. Effects of aging on glucose and lipid metabolism in mice. Aging Cell:e14462.
El Jarkass HT, Castelblanco S, Kaur M, Wan C, Ellis AE, Sheldon RD, Lien EC, Burton NO, Wright GD, Reinke AW. Pre-print. The Caenorhabditis elegans bacterial microbiome influences microsporidia infection through nutrient limitation and inhibiting parasite invasion. bioRxiv.
Sokol KH, Lee CJ, Rogers TJ, Waldhart A, Ellis AE, Madireddy S, Daniels SR, House RJ, Ye X, Olsenavich M, Johnson A, Furness BR, Sheldon RD, Lien EC. 2024. Lipid availability influences ferroptosis sensitivity in cancer cells by regulating polyunsaturated fatty acid trafficking. Cell Chem Biol.
House RJ, Tovar EA, Redlon LN, Essenburg CJ, Dischinger PS, Ellis AE, Beddows I, Sheldon RD, Lien EC, Graveel CR, Steensma MR. 2024. NF1 deficiency drives metabolic reprogramming in ER+ breast cancer. Mol Metab 80:101876.
2023
Jeong YJ, Rogers TJ, Anderson CE, Lien EC. 2023. Tumor lipid metabolism: a mechanistic link between diet and cancer progression. Curr Opin Biotech 84:102993.
2022
Li Z, Ji BW, Dixit PD, Lien EC, Tchourine K, Hosios AM, Abbott KL, Westermark AM, Gorodetsky EF, Sullivan LB, Vander Heiden MG, Vitkup D. 2022. Cancer cells depend on environmental lipids for proliferation when electron acceptors are limited. Nat Metab.
Gouirand V, Gicquel T, Lien EC, Da Costa Q, Finetti P, Mayers JR, Camoin L, Phphillat M, Audebert S, Borge L, Barea D, Rubis M, Leca J, Nigri J, Bertucci F, Birnbaum D, Iovanna JL, Tomasini R, Bidaut G, Guillaumond F, Vander Heiden MG, Vasseur S. 2022. Ketogenic HMG-CoA and its product β-hydroxybutyrate promote pancreatic cancer progression. EMBO J 41:e110466.
2021
Lien EC, Westermark AM, Zhang Y, Yuan C, Li Z, Lau AN, Sapp KM, Wolpin BM, Vander Heiden MG. 2021. Low glycaemic diets alter lipid metabolism to influence tumour growth. Nature 599:302–307.
2020
Chatterjee N, Whitman MA, Harris CA, Min SM, Jonas O, Lien EC, Luengo A, Vander Heiden MG, Hong J, Zhou P, Hemann MT, Walker GC. 2020. REV1 inhibitor JH-RE-06 enhances tumor cell response to chemotherapy by triggering senescence hallmarks. Proc Natl Acad Sci U S A 117(46):28918–28921.
Lau AN, Li Z, Danai LV, Westermark AM, Darnell AM, Ferreira R, Gocheva V, Sivanand S, Lien EC, Sapp KM, Mayers JR, Biffi G, Chin CR, Davidson SM, Tuveson DA, Jacks T, Matheson NJ, Yilmaz O, Vander Heiden MG. 2020. Dissecting cell type-specific metabolism in pancreatic ductal adenocarcinoma. Elife 9:e56782.
2018
Cox AG, Tsomides A, Yimlamai D, Hwang KL, Miesfeld J, Galli GG, Fowl BH, Fort M, Ma KY, Sullivan MR, Hosios AM, Snay E, Yuan M, Brown KK, Lien EC, Chhangawala S, Steinhauser ML, Asara JM, Houvras Y, Link B, Vander Heiden MG, Camargo FD, Goessling W. 2018. Yap regulates glucose utilization and sustains nucleotide synthesis to enable organ growth. EMBO J 37(22):pii: e100294.
Danai LV, Babic A, Rosenthal MH, Dennstedt EA, Muir A, Lien EC, Mayers JR, Tai K, Lau AN, Jones-Sali P, Prado CM, Petersen GM, Takahashi N, Sugimoto M, Yeh JJ, Lopez N, Bardeesy N, Fernandez-Del Castillo C, Liss AS, Koong AC, Bui J, Yuan C, Welch MW, Brais LK, Kulke MH, Dennis C, Clish CB, Wolpin BM, Vander Heiden MG. 2018. Altered exocrine function can drive adipose wasting in early pancreatic cancer. Nature 558(7711):600–604.
Hosios AM, Li Z, Lien EC, Heiden MVG. 2018. Preparation of lipid-stripped serum for the study of lipid metabolism in cell culture. Bio Protoc 8(11):e2876.
2017
Lien EC, Ghisolfi L, Geck RC, Asara JM, Toker A. 2017. Oncogenic PI3K promotes methionine dependency in breast cancer cells through the cystine-glutamate antiporter xCT. Sci Signal 10(510):eaao6604.
Shimizu K, Fukushima H, Ogura K, Lien EC, Nihira NT, Zhang J, North BJ, Guo A, Nagashima K, Nakagawa T, Hoshikawa S, Watahiki A, Okabe K, Yamada A, Toker A, Asara JM, Fukumoto S, Nakayama KI, Nakayama K, Inuzuka H, Wei W. 2017. The SCFβ-TRCP E3 ubiquitin ligase complex targets Lipin1 for ubiquitination and degradation to promote hepatic lipogenesis. Sci Signal 10(460):eaah4117.
2016
Cox AG, Tsomides A, Kim AJ, Saunders D, Hwang KL, Evason KJ, Heidel J, Brown KK, Yuan M, Lien EC, Lee BC, Nissim S, Dickinson B, Chhangawala S, Chang CJ, Asara JM, Houvras Y, Gladyshev VN, Goessling W. 2016. Selenoprotein H is an essential regulator of redox homeostasis that cooperates with p53 in development and tumorigenesis. Proc Natl Acad Sci U S A 113(38):E5562–E5571.
Cox AG, Hwang KL, Brown KK, Evason KJ, Beltz S, Tsomides A, O’connor K, Galli GG, Yimlamai D, Chhangawala S, Yuan M, Lien EC, Wucherpfennig J, Nissim S, Minami A, Cohen DE, Camargo FD, Asara JM, Houvras Y, Stainier DY, Goessling W. 2016. Yap reprograms glutamine metabolism to increase nucleotide biosynthesis and enable liver growth. Nat Cell Biol 18(8):886–896.
Juvekar A, Hu H, Yadegarynia S, Lyssiotis CA, Ullas S, Lien EC, Bellinger G, Son J, Hok RC, Seth P, Daly MB, Kim B, Scully R, Asara JM, Cantley LC, Wulf GM. 2016. Phosphoinositide 3-kinase inhibitors induce DNA damage through nucleoside depletion. Proc Natl Acad Sci U S A 113(30):E4338–E4347.
Zhang J, Xu K, Liu P, Geng Y, Wang B, Gan W, Guo J, Wu F, Chin YR, Berrios C, Lien EC, Toker A, Decaprio JA, Sicinski P, Wei W. 2016. Inhibition of Rb phosphorylation leads to mTORC2-mediated activation of Akt. Mol Cell 62(6):929–942.
Lien EC, Lyssiotis CA, Juvekar A, Hu H, Asara JM, Cantley LC, Toker A. 2016. Glutathione biosynthesis is a metabolic vulnerability in PI(3)K/Akt-driven breast cancer. Nat Cell Biol 18(5):572–578.
Mancini ML, Lien EC, Toker A. 2016. Oncogenic AKT1(E17K) mutation induces mammary hyperplasia but prevents HER2-driven tumorigenesis. Oncotarget 7(14):17301–17313.
Hu H, Juvekar A, Lyssiotis CA, Lien EC, Albeck JG, Oh D, Varma G, Hung YP, Ullas S, Lauring J, Seth P, Lundquist MR, Tolan DR, Grant AK, Needleman DJ, Asara JM, Cantley LC, Wulf GM. 2016. Phosphoinositide 3-Kinase regulates glycolysis through mobilization of aldolase from the actin cytoskeleton. Cell 164(3):433–446.
2014
Cuiffo BG, Campagne A, Bell GW, Lembo A, Orso F, Lien EC, Bhasin MK, Raimo M, Hanson SE, Marusyk A, El-Ashry D, Hematti P, Polyak K, Mechta-Grigoriou F, Mariani O, Volinia S, Vincent-Salomon A, Taverna D, Karnoub AE. 2014. MSC-regulated microRNAs converge on the transcription factor FOXP2 and promote breast cancer metastasis. Cell Stem Cell 15(6):762–774.
2013
Lien EC, Nagiec MJ, Dohlman HG. 2013. Proper protein glycosylation promotes mitogen-activated protein kinase signal fidelity. Biochemistry 52(1):115–124.


Addy Alexopoulos
Assistant Research Technician, Department of Metabolism and Nutritional Programming


Rachel (Rae) House, Ph.D.
Postdoctoral Fellow, Lien Laboratory
Thesis: Identification and exploitation of metabolic reprogramming in NF1-mutant breast cancer

Jared Fallon
Assistant Research Technician, Department of Metabolism and Nutritional Programming



Thomas Rogers, Ph.D.
Research Scientist, Department of Metabolism and Nutritional Programming

Althea Waldhart, B.S.
Senior Research Technician, Department of Metabolism and Nutritional Programming

Jeanie Wedberg
Senior Administrative Assistant II

